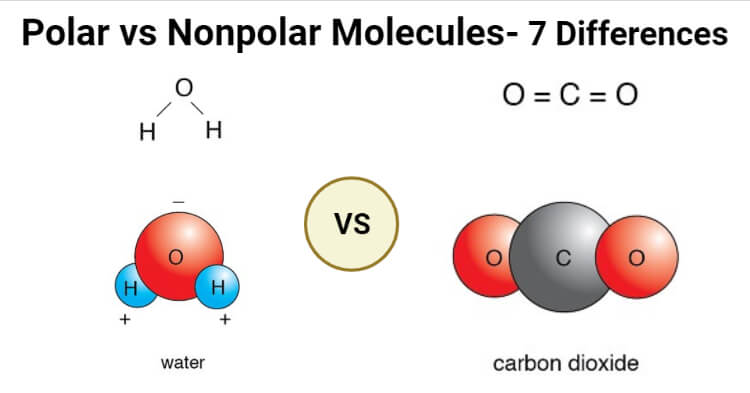
Polar molecules and nonpolar molecules are the two basic types of molecules. Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. Here’s an explanation of what polar and nonpolar imply, how to predict if a molecule will be one or the other, and examples of distinguishing molecules. Electronegativity is an atom’s capacity to attract electrons when they are in a compound. The periodic table below depicts the electronegativity of elements. The difference in electronegativity between the two atoms involved in the bond is used to assess whether a specific bond is ionic or covalent.
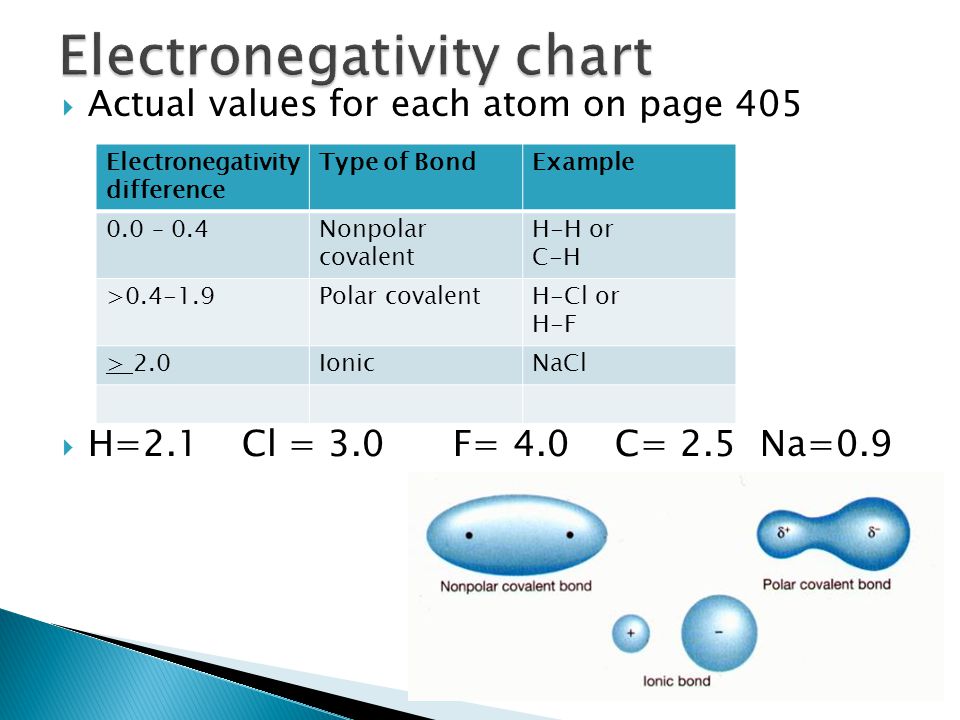
Consider the connection that exists between a potassium atom and a fluorine atom as an example. Using the table, the electronegativity difference is equal to 4.0 – 0.8 = 3.2. The connection between the two atoms is ionic because the difference in electronegativity is rather substantial. Because the fluorine atom attracts electrons considerably more strongly than the potassium atom, the valence electron from the potassium atom is entirely transferred to the fluorine atom. The figure below depicts how differences in electronegativity relate to whether a chemical bond is ionic or covalent.

Characteristics of Polar and nonpolar bonds
Nonpolar Bonds Characteristics
- When atoms share a pair of electrons, the result is a nonpolar covalent connection.
- Charge separation between linked atoms is not possible with a nonpolar covalent connection.
- Atoms of the same element create a nonpolar covalent bond.
- Nonpolar covalent bonds are powerful bonds that take a lot of energy to break.
- Because nonpolar covalent bonds have no interaction or polarity, they have a lower melting and boiling point than nonpolar covalent bonds.
- Because they lack charged particles, molecules with nonpolar covalent bonds do not conduct electricity.
- Nonpolar covalent links make molecules insoluble or less soluble in water. Nonpolar solvents such as CCl4, CHCl3, and others, on the other hand, make them more soluble.
- The dipole moment of molecules having polar covalent bonds is zero.
Polar Bonds Characteristics
Polar covalent bonding is a chemical connection in which two atoms share a pair of electrons unequally.
- The creation of opposing partial charges on the connected atoms, known as a dipole, is the consequence of a polar covalent bond.
- Atoms of different elements create a polar covalent connection.
- When compared to nonpolar covalent bonds, polar covalent bonds are comparatively weak.
- The melting and boiling points of polar covalent bonds are higher than those of nonpolar covalent bonds.
- Due to the unrestricted mobility of ions, molecules with polar covalent bonds transmit electricity in solution.
- In polar solvents like water, molecules having polar covalent bonds are highly soluble.
- Permanent dipole moment exists in molecules having polar covalent connections.
- Water (H2O), Hydrogen Fluoride (HF), and ammonia are examples of molecules possessing polar covalent bonds (NH3).

Polar and Nonpolar Covalent Bonds Differences
Differences in electronegativity and electropositivity
The electronegativity differential between the linked atoms in the bond allows them to spend a fair amount of time with electrons in a nonpolar molecule. In a polar molecule, however, there is no fair share. Consider the water molecule H2O, and it is the opposite. In the bond, oxygen is more electronegative than hydrogen. Electronegativity is the magnitude to which an atom requires electrons to complete an octet or duet. But the electrons spend more time with Oxygen than Hydrogen, making water bipolar.
Thus we consider electronegativity differences as a “rule of thumb.” This is here to determine whether a bond will be covalent, polar covalent, or ionic.
Differences in Bond formation
Polar bonds form between atoms of different elements. But, nonpolar bonds form between atoms of the same stuff.
Polar molecules are more reactive than nonpolar ones.
This is just a result of the minor charges they’d face (with a nonpolar molecule non, no charges). So you’ve got specific molecules that don’t dissolve in water, and you’ve got an organic molecule (a lipid ). A saturated butter has no net charges and is saturated (every atom on it is complete). As a result, it is incapable of reacting with solvents.
Differences in boiling and melting point
The melting and boiling points of polar covalent bonds are higher than those of nonpolar covalent bonds. Nonpolar covalent bonds have a lower melting point and boiling point than nonpolar covalent bonds. This is because they have no interaction or polarity.
Differences in conductivity
Due to the unrestricted mobility of ions, molecules with polar covalent bonds transmit electricity in solution. Because they lack charged particles, molecules with nonpolar covalent bonds do not conduct electricity.
Polar and Nonpolar Covalent Bonds Example
Polar Covalent Bonds:
- Water – H2O
- Hydrogen sulfide – H2S
- Ethanol – C2H6O
- Ammonia – NH3
- Sulfur dioxide – SO2
Nonpolar Covalent Bonds
- Carbon tetrachloride – CCl4
- Methane – CH4
- Ethylene – C2H4
- Carbon dioxide – CO2
- Benzene – C6H6
Polar and Nonpolar Covalent Bonds Explained
Polar covalent bond
The electrons in a polar covalent connection are not evenly shared because an atom spends more time with electrons than the other one. One atom has a greater pull than the other and draws electrons in polar covalent connections. Do you remember how electrons have a negative charge? When electrons spend more time with one atom, that atom gains a partial negative control. The atom that spends less time with the electrons has a partial positive charge. To recall a polar covalent bond, say ‘puller covalent,’ and keep in mind that one atom has a more incredible ‘pull’ on electrons than the other.
Examples
A polar covalent bond is represented by the water molecule, written as H2O. The electrons are distributed unequally, with the oxygen atom spending more time with them than the hydrogen atoms. The oxygen atom has a partial negative charge because electrons spend more time with it.
Read Also: Differences Between Naturalism and Realism in Literature: Definitions, Characteristics & Examples
We can also form a polar covalent bond between a hydrogen atom and a chlorine atom. The chlorine atom spends more time with the electrons in this connection than the hydrogen atom. Because of this uneven electron sharing, the chlorine atom has a partial negative charge while the hydrogen atom has a partial positive charge.
Nonpolar covalent bond
When two sister atoms share a pair of electrons, the result is a nonpolar covalent connection. Two or more bits are joined together by these shared electrons to create a molecule in a nonpolar covalent link exchange electrons in the same way children share toys. The link between two hydrogen atoms is a nonpolar covalent bond since they exchange electrons evenly. In biology, nonpolar covalent connections are highly essential. They assist in making up our living cells and generate the oxygen we breathe. A peptide bond is a type of nonpolar covalent link that is highly essential in biology. A peptide bond is a chemical connection that connects chains of amino acids used to make proteins. Carbon, oxygen, nitrogen, and hydrogen are among the elements that make up amino acids.
Example
The link between two chlorine atoms is an example of a nonpolar covalent bond since they exchange electrons evenly. Nonpolar covalent bonds are extremely strong bonds that take a lot of energy to break.
Polar and Nonpolar Covalent Bonds Electronegativity
Even though we are considering covalent bonding as electron sharing, electrons in a covalent bond are not always sharing electrons evenly by the two connected atoms. Unless the connection joins two bits of the same element, one atom will always attract the electrons in the bond more strongly than the other. When such an imbalance develops, some negative charge accumulates on one side of the bond. And some positive charge gets on the opposite side of the bond. A polar covalent bond is one in which the electrons are staying unequally. A nonpolar covalent bond is the one in which the electrons share themselves equally.
How to predict Covalent Bonds using electronegativity
You now might be wondering how you can predict the sort of connection that will form between atoms. The electronegativity of atoms involved in a bond can forecast which kind of bond will develop. The strength with which one bit attracts electrons from another atom in a chemical connection is electronegativity.
Electronegativity differences are popular as a “rule of thumb”. It determines whether a bond will be covalent, polar covalent, or ionic. If the difference in electronegativity between two linking atoms is smaller than 1/2, the bond is then a covalent bond. When it gets more than 2, the more electronegative atom entirely removes a valence electron from the electropositive atom. Thus, it creates an ionic compound. If it remains between 0.5 and 2.0, the polar covalent compound creates itself.
We may determine that a bond is polar if the difference in electronegativity between the atoms of the bond is between 0.5 and 2.0. If the particle is a diatomic, the molecule must be polar.
When a distance separates two equal charges, they form an electric dipole. The dipole moment is also a vector. This measures the intensity and orientation of an electric dipole and is measured in Debye units. The charges of the polar bond and the distance between them determine the dipole moment of a molecule. Without taking into account the vector characteristics, this may be computed using Equation:
μ=qr(8.7.2)