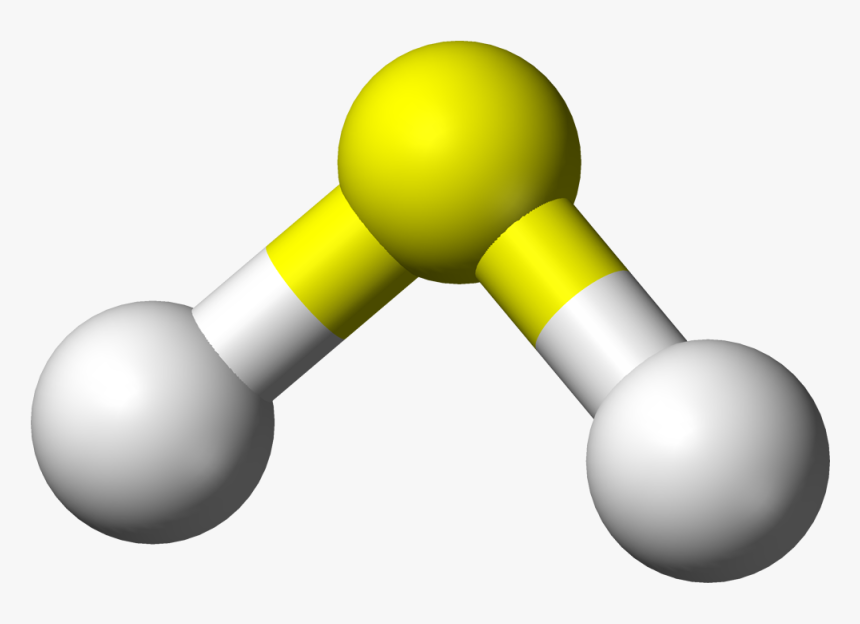
We know hydrosulfuric acid more popularly as hydrogen sulfide gas. The formula of hydrosulfuric acid is H2S. This is the gas with the smell of a rotten egg. We have all encountered this some or the other time in your chemistry laboratories at school.

What is hydrosulfuric acid?
Hydrogen sulfide is an acidic chemical compound. It has a pH of 4.5. Along with the characteristic foul smell of rotten eggs, it is toxic, can cause corrosion and fires. As a chalcogen hybrid gas, it shows no coloration. Microbial degradation of organic wastes in a limited supply of oxygen in marshy lands is one of its biggest sources. This they call anaerobic digestion. The microbes which can perform this breakdown are sulfate-reducing. Volcanic gases, natural gas,and some specimens of well water also contain it.
H2S exists even in the human body in small traces. It acts as a beckoning molecule there. In 1777, the Swedish scientist Carl Wilhelm Scheele decided on the chemical nature of H2S. The Brits spell it as “sulfide.” However, the International Union of Pure and Applied Chemistry (IUPAC) or even the Royal society of chemistry does not accept this spelling. They write “sulphide.”

Is Hydrosulfuric acid strong or weak, ionic or covalent?
Hydrosulfuric acid has a chemical structure that is the same as that of water. However, oxygen is much more electronegative than sulfur. So, this makes H2S much less polar compared to water. Hence, in H2S, only weak intermolecular forces exist against the strong ionic forces of water. So, it is much less polar compared to water. Therefore, it is a weak acid, and covalent bonds exist between the molecules due to lack of polarisation. Therefore, the boiling and melting points of H2S are also way lesser than that of water. While the water boils at a temperature of 100 degrees celsius, H2S does so at a temperature of -60.7 degrees celsius.
How is hydrosulfuric acid created?
Hydrosulfuric acid contains two atoms of Hydrogen and one atom of Sulphur. Hydrogen sulfide has a density that is much more than that of the atmospheric air. When hydrosulfuric acid or hydrogen sulfide (H2S) reacts with oxygen, it gives off a blue flame. The flame test often acts as an identification test for the gas. On self-combustion, it releases water and sulfur dioxide gas. It is also a good reducing agent. A reducing agent is a compound or an element that releases electrons to a different element or compound during a redox reaction.
What is the pH of hydrosulfuric acid?
H2S is a weak acid. It has a pH of around 5, while the neutral pH of water is 7.
Hydrosulfuric acid vs. sulfuric acid?
Is hydrosulfuric acid and sulfuric acid same? No, they are not. Hydrosulfuric acid consists of two hydrogen atoms and one sulfur atom. Its chemical formula is H2S. On the other hand, sulfuric acid consists of two hydrogen atoms with a sulfate ion. This makes it highly electronegative and is hence a very strong acid. Its chemical formula is H2SO4.
What is the symbol of Hydrosulfuric acid?
The molecular formula of Hydrosulfuric acid is H2S.
What are the features of hydrosulfuric acid?
Below we will see some of the key features and reactions of hydrosulfuric acid.
How are catalysts important in reactions involving hydrosulfuric acid?
Catalysts are substances that increase the rate of a reaction. They do not react with the reactants. Catalysts do not tamper with the final products too. Hydrosulfuric acid and sulfur dioxide react with each other at high temperatures in the presence of a catalyst. Water and sulfur are the products of this chemical reaction. In industrial operations, this process finds wide usage to process hydrogen sulfide. It acts as a weak acid. H2S is also soluble in water. However, it is not extremely well-soluble in water. This happens because, on combustion in the presence of air, it oxidizes to form sulfur. Sulfur is not water-soluble. So, it retains some amount of insolubility.

How does hydrosulfuric acid look?
Hydrosulfuric acid in the pure state has no color. Therefore, its solutions are also colorless. However, it reacts with metal ions. Such reactions form metal sulfides that are not colorless. The metal sulfides are generally dark in color. They are also insoluble in water. These metal sulfides can release hydrogen sulfide when chemists make them react with very strong acids.
When can hydrosulfuric acid conduct electricity?
The molecules of hydrosulfuric acid can conduct electricity. However, the necessary condition is high pressure, well above 90 gigapascals. H2S, when it is in the exposition of very high pressure, shows signs of superconductivity too. However, along with high pressure, chemists need to cool it down to extremely low temperatures as well. The temperature should be lower than the critical temperature. With an increase in pressure, the critical temperature rises as well. This critical temperature for H2S is somewhat 23K at a pressure of 100 gigapascals and 150K when the pressure is 200 gigapascals.
How potent is the odor of hydrosulfuric acid?
The odor of Hydrosulfuric acid is extremely potent. One can detect it at levels as much as 2ppb. Suppose, one ml of the gas gets distributed in a lecture hall of a college that can accommodate 200 students. So, the space is huge. However, it would still show a concentration of 20 ppb.

What is the easiest way to obtain hydrosulfuric acid?
The easiest way to get it is by separating it from the “Sour gas.” Sour gas is a natural gas. It contains a high amount of H2S gas. So, as one can guess, the process of obtaining H2S becomes very easy.
Read Also: Continuous Spectrum: Definition & Overview
There is another way too. We can make hydrogen gas react with molten sulfur at the nascent state. This sulfur should be monatomic. We need to make sure that the temperature is somewhere around 450 degrees celsius. Hydrocarbons might be very useful sources of hydrogen in this case. The resulting product would be H2S. H2S will form hydrosulfuric acid when we dissolve it in water. Both have the same composition. However, when we use the term acid, we choose to mean the liquid state. This is even though both are acidic in nature.
What do you mean by the aqueous solution of Hydrosulfuric acid?
What we have been talking about mostly is hydrogen sulfide gas. When we dissolve this gas into water, the resulting solution formed is the acid, or as chemists call it, the aqueous solution. In an aqueous solution, the constituent ions attain an equilibrium.
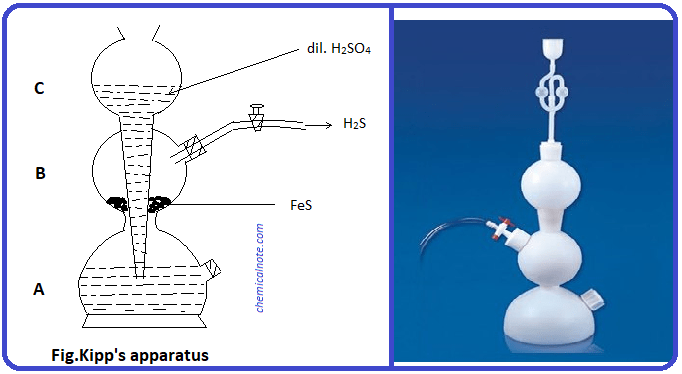
How is hydrosulfuric acid prepared in the laboratory?
Generally, in the laboratory, chemists use a Kip generator. Inside this, they react ferrous sulfide with a very strong acid. This forms ferrous chloride and releases hydrogen sulfide gas. The standard equation for lab preparation is-
FeS + 2 HCl → FeCl2 + H2S
This is then dissolved in water to form the acid.
What are a few other ways to prepare Hydrosulfuric acid?
In the case of qualitative inorganic analysis, chemists tend to use thioacetamide and react it with water. This releases Hydrogen sulphide gas. It can dissolve in water to form acid.
The equation of H2S formation from thioacetamide is-
CH3C(S)NH2 + H2O → CH3C(O)NH2 + H2S
Various sulfides of both metals and non-metals too act as sources of hydrogen sulfide. They release the gas when they react with water. Examples of such sulfides are aluminium sulfide (metal sulfides), phosphorus pentasulphide, silicon disulfide (non-metal sulfides), etc.
For example, the equation of preparation from aluminium sulfide is-
6 H2O + Al2S3 → 3 H2S + 2 Al(OH)3
Chemists can also easily form the gas by heating the sulfur with solid organic compounds. They can also form it by reducing organic compounds containing Sulphur in the presence of hydrogen gas. This is because hydrogen is an excellent reducing agent.




