What Is Formal Charge?
Whenever we study our accounts, it is right to recognize if we’re manner over budget (terrible), simply right (neutral), or if we are able to manage to pay for to spend greater (positive). It’s the equal manner within side the subatomic degree while we study electrons shared among atoms in a molecule. An atom will have the subsequent costs: positive, terrible, or neutral, relying at the electron distribution. Just like we are able to calculate if our account is positive, terrible, or balanced, there may be a manner to calculate an atom’s rate in a molecule.
We can decide what the electron distribution in a molecule is with the aid of using identifying the formal rate. The formal rate is the rate of an atom in a molecule. By including all the formal costs of all the atoms within side the molecule, you could decide if the general rate of the molecule is positive, terrible, or neutral. The manner to decide the formal rate is with the aid of using the use of the subsequent equation.
Lewis Structure
To visualize what the method says, we will first study the Lewis Structure, that’s a drawing of the molecule that suggests all of the bonding and nonbonding electrons. Let us check the instance of the carbon dioxide Lewis Structure proven here.
bonding electrons, we want to be counted number the traces at the structure. One line corresponds to 2 electrons. The nonbonding electrons, on the opposite hand, are the unshared electrons. These are proven as dots. One dot is identical to at least one nonbonding electron. The valence electrons are the electrons at the outermost shell of the atom.
Valence Electrons
Let’s do not forget a easy manner to be counted number valence electrons primarily based totally on their organization quantity within side the periodic table. There is a sample for counting valence electrons primarily based totally at the organization quantity an atom has. For example, sodium (Na) is in organization 1, consequently, it has 1 valence electron. Oxygen (O) is in organization 16, consequently it has 6 valence electrons. Bromine (Br) is in organization 17, so it has 7 valence electrons. An exception that we want to be aware of is for Helium (He).
It is in organization 18; however, it has best 2 valence electrons due to the fact its most quantity of electrons is 2. All the opposite noble gases in organization 18, together with Neon (Ne), Argon (Ar), and the rest, have eight valence electrons. We overlooked companies 3-12. That’s due to the fact no sample exists for the valence electrons for those elements, and there may be a one-of-a-kind and greater complicated manner of counting their valence electrons.
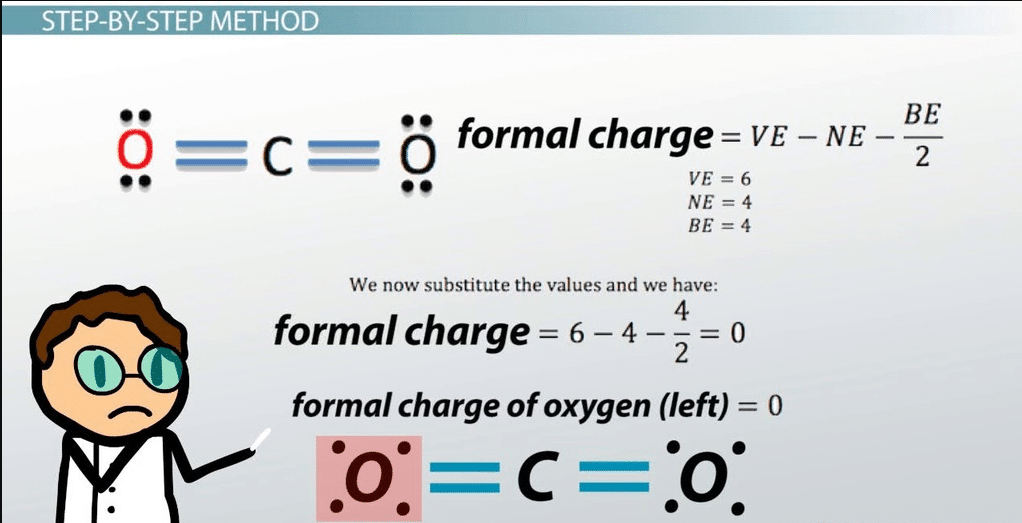
Step-By-Step Method
Now that we recognize the method for figuring out the formal rate, let’s placed this into exercise and move over a way to decide the formal rate for every atom in a molecule step with the aid of using step. The method for the formal rate is:
Here’s a question. Alkanes, alkenes, and alkynes are neutral, considering there are 4 bonds and no unbonded electrons: 4 – [4+0] = 0. For what different values of [bonds + nonbonded electrons] will you furthermore mght get a fee of zero, and what may those systems appearance like? (You’ll meet a number of those systems later within side the course).
One very last question – why do you observed that is called “formal rate”?
Think approximately what the formal rate of BF4 might be. Negative rate at the boron. What’s the maximum electronegative detail here? Fluoride, of course, with an electronegativity of 4.0, with boron clocking in at 2.0. Where do you observed that terrible rate really resides?
Well, it ain’t on boron. It’s without a doubt unfold out via the greater electronegative fluoride ions, which turn out to be greater electron-rich. So even though the “formal” deal with of the terrible rate is on boron, the electron density is without a doubt unfold out over the fluorides. In different words, in this example the formal rate bears no resemblance to reality.




