In different words, ionic compounds held collectively via way of means of ionic bonds as classed as ionic compounds. Elements can benefit or lose electrons to be able to acquire their nearest noble gas configuration. Formation of ions (both via way of means of gaining or dropping electrons) for the of of entirety of octet facilitates them benefit stability. In a response among metals and non-metals, metals typically unfastened electrons to finish their octet even as non-metals benefit electrons to finish their octet. Metals and non-metals typically react to shape ionic compounds.
Ionic Compound Structure
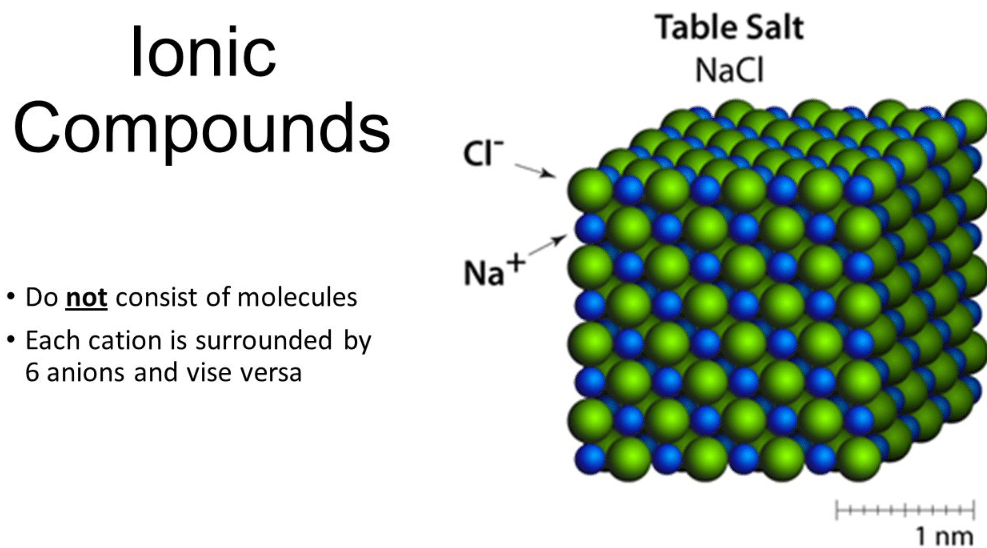
The shape of an ionic compound relies upon at the relative sizes of the cations and anions. Ionic compounds encompass salts, oxides, hydroxides, sulphides, and the bulk of inorganic compounds. Ionic solids are held collectively via way of means of the electrostatic appeal among the high-quality and poor ions. For instance, the sodium ions entice chloride ions and the chloride ion draws sodium ions. The end result is a three- d shape of exchange Na+ and Cl– ions. This is a crystal of sodium chloride.
The crystal is uncharged due to the fact the variety of sodium ions is identical to the variety of chloride ions. The forces of appeal among the ions preserve them within side the systems. These ionic bonds among the charged debris bring about a massive shape of ions. Because the ions are held collectively tightly in those massive systems it takes lots of power to interrupt all of the bonds. As a end result, ionic compounds have excessive melting factors and boiling factors.
Ionic Compound Examples
For instance response among magnesium and chlorine. The magnesium atom has electrons in its outermost shell. By dropping electrons from its M shell its L shell turns into the outermost shell that has a strong octet. The nucleus of this magnesium atom nonetheless has twelve protons however the variety of electrons has reduced to ten. So, a internet high-quality fee is evolved in this magnesium atom, giving a magnesium cation Mg2+.On the alternative hand, the chlorine atom has seven electrons in its outermost shell.
Therefore, it wishes handiest one electron to finish its octet. It can benefit this one electron from the electrons misplaced via way of means of magnesium atom to grow to be magnesium ion. As electrons are misplaced via way of means of magnesium atom. Even as one chlorine atom can benefit one electron. Atoms of chlorine integrate with one atom of magnesium to shape magnesium chloride.
From the above instance, ionic compounds may be described because the compounds shaped via way of means of the switch of electrons among metals and non-metals. The bond shaped among them is called the ionic bond. Due to the presence of oppositely charged ions, ionic compounds hold strongly via way of means of the electrostatic pressure of appeal. Prominent houses of ionic compounds are:
Ionic Compound Properties
1. Physical houses of ionic compounds
Due to the presence of the sturdy pressure of appeal among the high-quality and poor ions, ionic compounds are solids and are tough to interrupt. They typically ruin into portions while strain applies. Therefore, they may take into consideration brittle.
2. Melting and boiling factors of ionic compounds
Due to the presence of electrostatic forces of appeal among ions. A big quantity of power need to interrupt the ionic bonds among the atoms. Thus, ionic compounds have excessive melting and boiling factors.
3. The solubility of ionic compounds
Ionic compounds are typically soluble in polar solvents inclusive of water whereas solubility has a tendency to lower in non-polar solvents inclusive of petrol, gasoline, etc.
4. Conduction of Electricity
Ionic compounds do now no longer behavior energy within side the solid-nation however are appropriate conductors in a molten nation. Conduction of energy entails the float of fee from one factor to another. In the solid-nation, because the motion of ions isn’t possible, ionic compounds don’t behavior energy. Whereas within side the molten nation, ionic compounds behavior energy. As electrostatic forces of appeal among the ions are triumph over via way of means of the warmth released.




